Your specialist partner for breakthrough CNS and psychiatric research. Where rigorous science meets compassionate care.
We deliver complex CNS trials consistently, on time and within budget.
Clerkenwell Health exists to transform how clinical trials for pioneering CNS treatments are conducted and promoted. We're closing a critical gap in mental health research; millions suffer without effective treatments while life-changing clinical trials desperately search for participants. Our mission is to bridge that gap through specialist expertise, human-centered care, and purpose-built infrastructure.
We're leading a movement in clinical research
Brain and mental health research lags behind its impact on society. We reject the industry’s one-size-fits-all approach, building a system specifically engineered for the unique demands of CNS research. We’re not just adapting to the future of CNS trials – we’re defining it through our integrated approach that proves compassionate care delivers superior science.
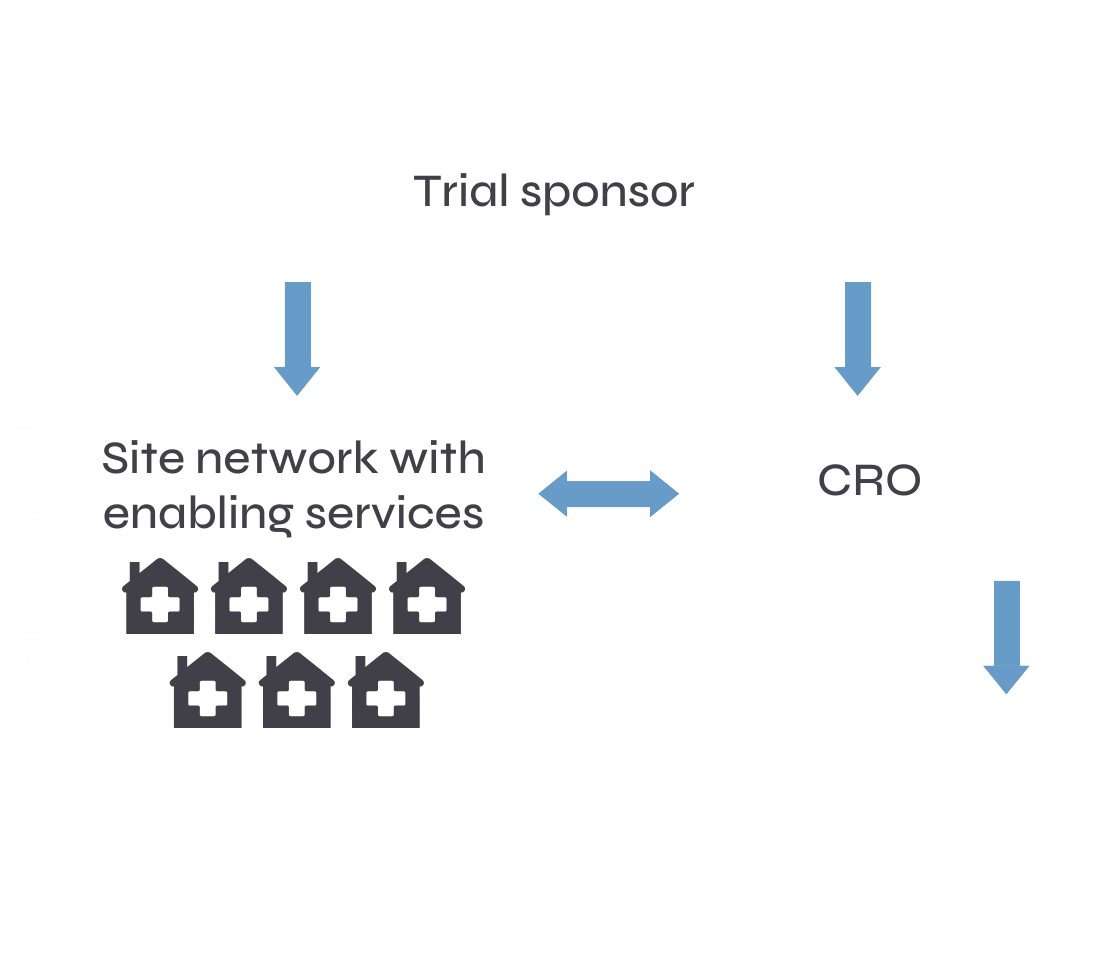
Integrated trial design and delivery
Our fully integrated model brings clarity and control to even the most complex CNS trials. By combining select CRO services, purpose-built trial sites, and advanced technologies under one system, we create seamless coordination that improves both speed and precision.
With a single contracting point across multiple sites and investigators, we simplify operations without losing sight of scientific or regulatory rigour. This joined-up approach allows us to design trials that are not only efficient to run, but also more supportive of participants. By sharing teams, tools and processes across every function, we deliver trials that move faster, feel better for patients, and generate stronger clinical outcomes.
People at the heart of every trial
Our approach to recruitment and delivery is grounded in years of experience in mental healthcare and research – always focused on the full journey, from first contact to final visit.
Using a flexible, multi-channel strategy, we quickly and cost-effectively find and screen the right participants. Our growing database of highly engaged patients is supported by strong relationships with charities, hospitals, clinicians, and therapists. This trusted network means we can build participant pipelines even before trials are approved – and expand to new sites without added regulatory delays.
3-10x
Faster recruitment compared to other sites
48h
Turnaround from sign-up to pre-screening call
90%
Enrolled participant retention
>50k
Vetted UK patient database
Fast site selection and trial set-up
Our team moves with speed and purpose – delivering faster feasibility assessments, onboarding and training to get trials up and running without delay. With a streamlined, structured approach to site management, we ensure operational excellence from day one, even for the most complex or specialist studies. We're also equipped to integrate emerging technologies and novel treatment approaches, making it easy to tailor trials to the needs of both science and patients.
2 wk
Between sponsor greenlight and first patient screened
2-3 wk
Between site selection and contracting
8-12 wk
Between site selection and first patient
Network of principal investigators and therapists
We’ve built a large network of principal investigators with deep expertise in CNS and psychiatry, supported by a pool of therapists trained to the highest clinical standards. This gives us the ability to quickly assemble the right team for each trial – no matter how specialised the requirements.
We understand that psychological support is critical to the success of psychedelic trials. That’s why we’ve developed a dedicated training programme for psychedelic practitioners, backed by robust supervision and monitoring. This ensures therapy is delivered safely, consistently, and in line with protocol – protecting both patient wellbeing and the quality of your trial data.
6
Investigators with experience in 20+ indications
>40
Trained therapists
>1k
practitioners in our database waiting to be trained
Deep expertise in psychiatry & CNS
Traditional CROs often apply generic, one-size-fits-all methods that miss the complexity and sensitivity of these patient populations. At Clerkenwell Health, we take a different approach – one built on deep understanding, clinical precision and genuine care.
Because we know that in mental health research, treating people with dignity and expertise isn't just the right thing to do – it's the key to breakthrough science that can transform millions of lives.
Our team combines scientific expertise with real-world insight to create protocols that are both regulatory-ready and patient-centred – improving recruitment, boosting retention, and delivering trials that work in practice, not just on paper.
15
Psychiatric indications we’ve worked with
100%
of clients said the work we delivered met or exceeded their expectation
100%
of clients think the work we delivered was good or excellent value
85.7%
of clients said they’d be working with us at the next opportunity
Our capabilities
-
Accelerated clinical development pathways
Regulatory advisory
Innovative trial design & protocol development
Support across phase I-III trial design
Focus on operational aspects to ensure optimised delivery
-
FDA, MHRA & EMA approvals
Full submission dossiers
Import / export support
Manage entire submission process
Lead regulator meetings
-
Site set-up & management
Biostatistics & data management
Trial performance management & monitoring
Clerkenwell-owned trial units
Global network of sites proven to deliver
-
Provided as stand-alone service or as part of site delivery
Multi-channel, tech enabled approach
Study-specific approach to deliver precision recruitment strategies
-
Market leading approach to recruiting, training & monitoring therapists in trial context
Ability to ‘white label’ therapy manuals
Processes to monitor and continually improve therapists practice and adherence to manuals